

You experience "science" everyday and everywhere. Your home is the best place to learn and think about "science". In the kitchen, we are familiar with the cookware and the cooking methods, the food flavor, texture and its storage, etc.; however, the science behind those things are not clear in mind. You are, sometimes, puzzled about some experience in the kitchen. In this chapter, you will have a better understanding on those mind-blowing questions.
Boiled and Raw Eggs |
|---|

The Hot Pizza |
|---|
 A hot pizza just out of the oven will burn your tongue more severe when you bite the fruit filling.
The much drier crust in the pizza will burn your tongue less severe.
Do you have such experience? The following paragraphs tell you the reasons about, and in addition
the key concepts in "temperature" and "heat" are introduced.
A hot pizza just out of the oven will burn your tongue more severe when you bite the fruit filling.
The much drier crust in the pizza will burn your tongue less severe.
Do you have such experience? The following paragraphs tell you the reasons about, and in addition
the key concepts in "temperature" and "heat" are introduced.
| How Things Work | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All ingredients in a hot pizza contain heat, but the amount of heat stored in each ingredient is not the same
though they are at the same temperature. A one-gram strawberry stores more heat than a piece of baked crisp
of the same weight, since strawberry contains high water content which has large "specific heat
capacity" (to be explained later). Or simply, water stores more heat than
other ingredients of the same weight under the same temperature increment. When you eat the wet portion of
a pizza, a large amount of heat flows and come to your month, the high temperature of the portion drops
finally to your body temperature, say 36.5oC.
You are then, of course, in a serious burn due to the large amount of heat that your mouth adsorbs in a short time.
Surely, the heat scalds you, but not the high temperature!
| ||||||||||||||||||||||||
| Insight | ||||||||||||||||||||||||
| For the same amount (you are familiar with the term "weight" or more technical "mass"),
of water and iron, say 1 kg, if one increases the temperature of these
substances by one degree Celsius (oC), the amount of heat that is further absorbed by water
is almost ten times that in iron.
Technically, we say that water has a higher "specific heat capacity" than iron, and water is an
efficient and common coolant as it absorbs heat from hot bodies without increasing its temperature greatly.
Poor coolant may escape away as its temperature increases rapidly and finally it boils and evaporates.
| ||||||||||||||||||||||||
| Science in Depth | ||||||||||||||||||||||||
There are few terms that are quite common in the discussion of heat.
|
The Thermostat |
|---|



| How Things Work | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Most substances expand when heated. Two metallic strips, with the same original lengths
but not of the same kinds, increase differently in lengths when they are heated up. The thermostat
is made up by a bimetallic strip which consists of two metals bonded together to form
a strip. Suppose metal A expand/shrunk less than metal B when heated/cooled. The figure below shows a
bimetallic strip which has metal A (e.g. iron) colored in black and metal B (e.g. copper) colored in brown.
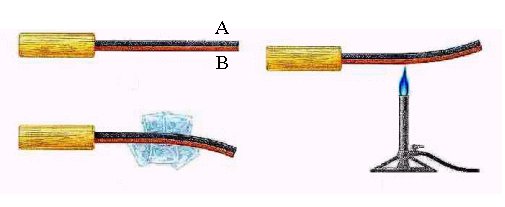 This means that its length will change by a greater amount than metal A for the same temperature change. Hence, if this strip is cooled, the B side will shrink more than A side, resulting in the strip bending toward the B side. On the other hand, if the strip is heated, the B side expands by a greater amount than the A side. Thus, the shape of the bimetallic strip depends sensitively on temperature. The bimetallic strip deflects in one direction or the other, which either closes or breaks the electrical circuit connected to the heater, as shown in the right figure below. Click the left figure for the movie on a bimetallic strip. (Credit: NCSU Physics Education Technology Facility)
| ||||||||||||||||
| Insight | ||||||||||||||||

 | ||||||||||||||||
| Science in Depth | ||||||||||||||||
|
Water Droplets on a Red-hot Wok |
|---|
 When a few water droplets are poured onto a red-hot wok, they run to and fro vigorously.
The wok seems a perfect surface and the droplets slide on it without hindering. Repeat the
whole process, but this time with a wok at room temperature. You observe nothing special.
When a few water droplets are poured onto a red-hot wok, they run to and fro vigorously.
The wok seems a perfect surface and the droplets slide on it without hindering. Repeat the
whole process, but this time with a wok at room temperature. You observe nothing special.
| How Things Work | ||
|---|---|---|
| ||
| Insight | ||
| The thermal expansion of water in the kitchen is a common scene in the kitchen.
The following example is familiar to you, but it is really dangerous.
Droplets of vegetable oil spill out in the kitchen when you pour small water droplets
into the oil at red-hot temperature. The reasons are straightforward.
The boiling point of oil is about 200 oC, which is much higher than that of
water, so water is heated up and it changes to steam before the boiling of oil. As the steam at
100 oC has a volume which is 1700 times that of liquid water in room temperature.
Droplets of oil spill out everywhere.
| ||
| Science in Depth | ||
| Information for your reference: We quite often use 18 g of water in our calculation, since one "mole" of water contains 18 g in mass and the term "mole" is a very useful quantity (unit) in chemistry. We regard one dozen to represent 12 objects in daily life, but in science, we adopt one mole to represent 6.02 × 1023 molecules. The volume of one mole of gas is fixed at 22.4 dm3 at 0 oC. At a higher temperature, say, 100 oC, one mole of gas has a volume of 30.6 dm3 after calculations. Remind that one mole of liquid water has 18 g in mass, thus has a volume of 18 cm3. The ratio in expansion, 1700 (mentioned in the last paragraph), is obtained readily if we divide 30.6 dm3 by 18 cm3. (Note: 1 dm3 = 1000 cm3). |
Pour the Ketchup |
|---|
 Sometimes you find the ketchup in the bottle hard to pour out from it when it is not shaked for a long time.
A "genius" (it could be your mother or father when you was young) comes and tells you to do the following.
Sometimes you find the ketchup in the bottle hard to pour out from it when it is not shaked for a long time.
A "genius" (it could be your mother or father when you was young) comes and tells you to do the following.
| How Things Work | |||
|---|---|---|---|
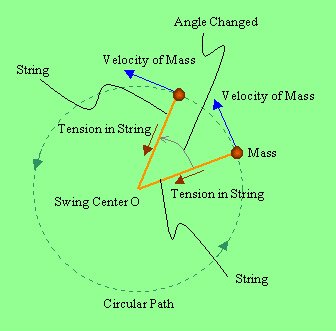 Imagine you swing a small mass which is attached to one end of a string. The
mass moves in a circular path and maintains a constant radial distance from your hand. Clearly,
the mass is pulled and constrained by a force (tension) which points toward your
hand (swing center) along the string (read more in "Science in Depth").
The figure in the right
describes the motion of the mass constrained in a horizontal circular path by a string.
Imagine you swing a small mass which is attached to one end of a string. The
mass moves in a circular path and maintains a constant radial distance from your hand. Clearly,
the mass is pulled and constrained by a force (tension) which points toward your
hand (swing center) along the string (read more in "Science in Depth").
The figure in the right
describes the motion of the mass constrained in a horizontal circular path by a string.
Now, let us think about the force on the ketchup droplet. Suppose every ketchup droplet moves with the swinging bottle while its radial distance from the bottom of bottle unchanged, then the possible force which works similar to the tension in the string-mass system should be the viscous force among droplets. However, this force is not great enough (we have no string now) to maintain the sauce droplet at its original position in the bottle. So, sauce pours out when it is moving with angular change. Specifically, the migration of sauce towards the lid of bottle is the result of the so called 'centrifugal (pointing away from the center) force' which is referred to in the view of the swinging bottle. However, the centrifugal force is regarded as a 'fictitious force' in the view of the person who are sitting outside the rotating bottle. | |||
| Insight | |||
 You will find the above trick quite often applied in daily life.
You will find the above trick quite often applied in daily life.
| |||
| Science in Depth | |||
|
Issac Newton (1642-1727), the greatest British scientist, announced his three primary
laws of motions.
The first law consider a mass on which no force acts. If the mass is at rest, it will remain at rest.
If the mass is moving with constant velocity, it will continue to do so.
This law is quite often described as the "law of inertia" and therefore it explains the
need of a force (tension) in the string-mass system, since the mass
ever changes it direction in a circular motion (see the figure above in the section "How Things Work").
The tension force in the string is specified as
"centripetal (pointing towards the center) force" which points toward the swing center. The mass is
pulled to change its direction while moving in a circular orbit.
When such force disappears (e.g. the string is broken)
the mass will no longer move in a circular path but instead a straight line.
The above discussion is basic and important in circular motion. Not surprisingly, you might struggle on its irrelevance to the case "swinging the ketchup bottle". Nevertheless, they are not isolated stories. As an illustration similar to swinging the ketchup bottle, the centrifugal motion is demonstrated in the below video on a merry-go-round. The weights leave the surface of the merry-go-round due to the rotation of the merry-go-round. Specifically, the merry-go-round is unlike the bottle which has bottle wall to govern the angular change of sauce droplet.
The centrifugal force is applied for a medical instrument, the centrifuge or the blood separator. This laboratory equipment is designed to separate molecules from solution, e.g. the red cells, white cells from the plasma in blood. A test tube of blood is placed into the centrifuge where it is spun at speeds of several thousand revolutions per minute (rpm). The denser red cells experience a greater centrifugal force than the lighter white cells, The red cells are pushed through the plasma, settling at the bottom of the tube. The white cells are buoyed up by plasma and gather in a distinct white band farther up the tube.
|
The Secret of "Simmer" |
|---|
 The Chinese healing soup is commonly prepared in families to cure minor complaints in
body or to improve general health. The ginseng cooker (a special soup cooker; "ginseng" is the same as "ren shen")
for "simmer" is
specially designed to produce rich soup. With a ginseng cooker, which has two lids, good soup essence
is not dissipated in steam. The cooking process is as follows.
Place a ginseng cooker inside a larger pot along with enough water covering the ingredients.
The pot is then filled with a large amount of water.
During the cooking process, the flavor and essence
of the ingredients are retained in the soup as water in the ginseng cooker never boils.
You might be puzzled by the situation that "it never boils" despite of the stove.
The Chinese healing soup is commonly prepared in families to cure minor complaints in
body or to improve general health. The ginseng cooker (a special soup cooker; "ginseng" is the same as "ren shen")
for "simmer" is
specially designed to produce rich soup. With a ginseng cooker, which has two lids, good soup essence
is not dissipated in steam. The cooking process is as follows.
Place a ginseng cooker inside a larger pot along with enough water covering the ingredients.
The pot is then filled with a large amount of water.
During the cooking process, the flavor and essence
of the ingredients are retained in the soup as water in the ginseng cooker never boils.
You might be puzzled by the situation that "it never boils" despite of the stove.


| How Things Work | ||||||
|---|---|---|---|---|---|---|
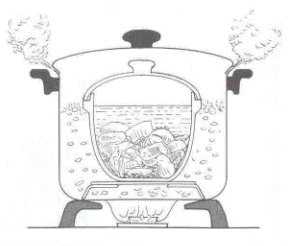 The soup (water) in the ginseng cooker
absorbs heat from the boiling water outside (i.e. the water in the pot) and reaches a
temperature 100 oC.
The ginseng cooker which is made of ceramic material acts as the medium to conduct heat in the cooking process.
Apparently, water needs extra heat (latent heat) to change itself from liquid state to steam, and such
heat is referred to as the latent heat of vaporization. However, we notice that the water in the ginseng
cooker and the pot is at the same temperature (100 oC), and it stops further thermal conduction.
Thus, water in the ginseng cooker never boils and the flavor
of soup never escapes from the cooker.
It is obvious that the flavor will lose when water evaporates in boiling.
Furthermore, the essences of soup, sometimes they are with medical uses,
are not spoiled during the simmering process.
The soup (water) in the ginseng cooker
absorbs heat from the boiling water outside (i.e. the water in the pot) and reaches a
temperature 100 oC.
The ginseng cooker which is made of ceramic material acts as the medium to conduct heat in the cooking process.
Apparently, water needs extra heat (latent heat) to change itself from liquid state to steam, and such
heat is referred to as the latent heat of vaporization. However, we notice that the water in the ginseng
cooker and the pot is at the same temperature (100 oC), and it stops further thermal conduction.
Thus, water in the ginseng cooker never boils and the flavor
of soup never escapes from the cooker.
It is obvious that the flavor will lose when water evaporates in boiling.
Furthermore, the essences of soup, sometimes they are with medical uses,
are not spoiled during the simmering process.
| ||||||
| Insight | ||||||
|
The Vacuum flask |
|---|
| How Things Work |
|---|
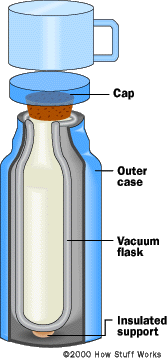
|
| Insight |
|
The Refrigerators |
|---|
| How Things Work |
|---|
 There are three states in matters, namely, the solid, liquid and gaseous states.
In everyday life, we know the three states of water as solid ice, liquid water and gaseous steam respectively.
The refrigerator works on the state changes of refrigerant,
e.g. between liquid and gaseous states, as heat transfer takes place in such process.
There are three states in matters, namely, the solid, liquid and gaseous states.
In everyday life, we know the three states of water as solid ice, liquid water and gaseous steam respectively.
The refrigerator works on the state changes of refrigerant,
e.g. between liquid and gaseous states, as heat transfer takes place in such process.
Convection currents carry cold air from the refrigerator, which is cooled to around 37oF. Internal temperature is controlled by a thermostat: this consists of a sealed, air-filled tube terminating in the refrigerator [5]. As air within the tube warms up, it expands, pushing out a set of bellows [6]. The expanding bellows close an electrical switch [7], which switches on the compressor. The refrigerator cabinet is made of polyurethane foam [8]: this functions as an insulator as well as giving mechanical strength. |
| Insight |
|
The Microwave Oven |
|---|
 Since humans learned how to create fire, heat has been used to process food. In conventional stoves, heat is
generated either by a controllable gas flame or by an electric heating element and is transferred to the food
by conduction, As over half the heat energy is lost to the environment by conventional appliance, stoves
have been developed that transfer energy more directly to the food. The most revolutionary of these
new cooking technologies is the microwave oven, which was patented in 1953, but early models were too
cumbersome for use in the home. Smaller and more efficient microwave ovens were developed in the 1970s,
and since then they have become increasingly popular both in homes and restaurants. The oven is said to be
unconvenional in cooking, as it cooks foods from within.
Since humans learned how to create fire, heat has been used to process food. In conventional stoves, heat is
generated either by a controllable gas flame or by an electric heating element and is transferred to the food
by conduction, As over half the heat energy is lost to the environment by conventional appliance, stoves
have been developed that transfer energy more directly to the food. The most revolutionary of these
new cooking technologies is the microwave oven, which was patented in 1953, but early models were too
cumbersome for use in the home. Smaller and more efficient microwave ovens were developed in the 1970s,
and since then they have become increasingly popular both in homes and restaurants. The oven is said to be
unconvenional in cooking, as it cooks foods from within.
| How Things Work |
|---|
|
| Insight |
Why Do We Heat Our Food?
|
The Superheat of Water in Microwave Oven |
|---|

|
|
You may guess that
the coffee powder lowers the boiling point of the water and hence
the water temperature would instantly be above the boiling point, and the water would begin to boil.
No! Absolutely not!!
It is the result of "superheating".
Superheating means the heating of a liquid to a temperature above its normal boiling point.
The superheated state is unstable, the liquid stays in its liquid state at or above the boiling point. However, it
rapidly turns into a substantial quantity of vapor when the unstable state collapses.
| How Things Work |
|---|
|
| Insight |
|
The Smell of Fish |
|---|
 We are all familiar with the smell of fish, however, freshly caught fish have no odor at all.
The "fishy" smell develops as chemical reactions take place in the flesh of the fish
sometime after they have been caught. The "fishy" smell cannot be eliminated completely even though the fish are
preserved under low temperatures. Do you know why?
We are all familiar with the smell of fish, however, freshly caught fish have no odor at all.
The "fishy" smell develops as chemical reactions take place in the flesh of the fish
sometime after they have been caught. The "fishy" smell cannot be eliminated completely even though the fish are
preserved under low temperatures. Do you know why?
| How Things Work |
|---|
|
| Insight |
| When you are buying fish it can be useful to remember that the fresher a fish, the less strong will be its smell. You should always eat fish as soon after it has been caught as is possible. Remember that the enzymatic reactions that can themselves consume the flesh cannot be delayed by refrigeration as they can for meats, so you need to eat the fish before its own enzymes eat it! |
The Color of Meats |
|---|
| How Things Work |
|---|

|
| Insight |
Some fish, notably, sharks and wild salmon are denser than the water in which they live; they literally have to
keep on swimming all the time to stay up. These fish need some slow fibers and in consequence have
darker meat than most other fish. The figure below shows the wild pacific chum salmon with red flesh.
 |